The cycle of PPI (Part I of II)
Ideally, you want to involve patients and the public as early as possible in the research process – if you want to maximise the impact of PPI (which you obviously do, seeing as you’re taking the time to read this post) then early is best. Going back to my house analogy (PPI introduction post) – it is a lot easier to get the ideal house if you are involved in the planning and construction phases than if you get brought in right at the very end. Sure, you can make sure that the walls are painted in the right colour, but that might be little consolation if the layout of the house isn’t right. That being said, and I cannot emphasise this enough, PPI at any stage is better than none at all.
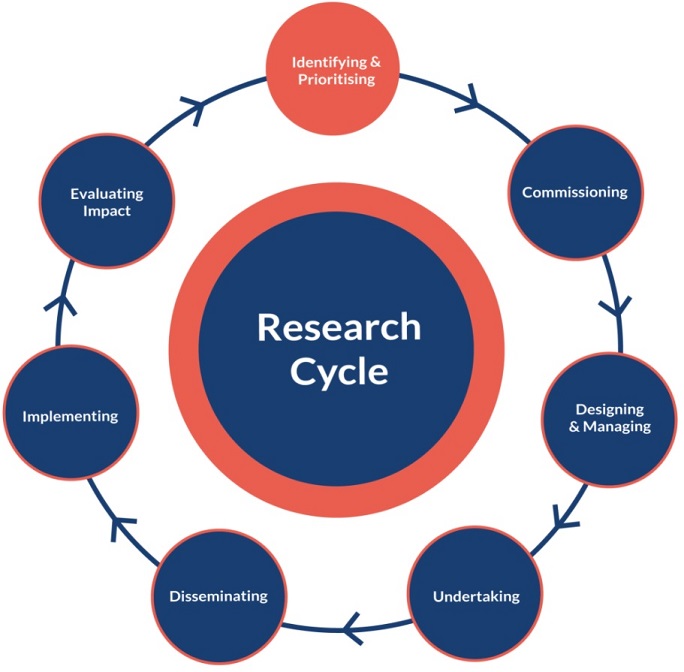
Delving into the question of WHEN, I find it is easiest to start at the very beginning. But when is the “beginning” in the realm of research? Let us instead, for simplicities sake, start at the very top of the National Institute of Health Research cycle.
If you were told that you had 3 million SEK to fund research, with your only guideline being that it has to be within a certain disease area – how would you go about deciding what research to fund? My approach would be to ask the people affected by the disease what they need.
With that, we start with identifying and prioritising research – the funding bodies’ territory. The members of the public have a large part to play in identifying and prioritising the topics that the funding bodies should support. The difficulty here is how to elicit the priorities from the public – being asked to list the research priorities in one’s disease area can be quite daunting for people unfamiliar with research. Instead, ask what their problems are and then use your expertise to convert these into research questions. An example of a formalised process for this the Priority Setting Partnerships by the James Lind Alliance. I was fortunate enough to see the inner workings of one aimed at blistering skin diseases – fascinating, informative, but resource-intense. The good news is that you can involve patients and the public on a much smaller and simpler scale – and that is exactly what is on my to-do list at the moment. I am creating an online survey that will be distributed to people with a personal experience of a certain diagnosis, asking what their top three questions or concerns are about their disease. Hopefully what will emerge are themes, recurring questions, that we can shape into funding calls.
You put out the funding call and received 20 applications, but can only fund 10. Who do you involve in the grant review process? Clinicians, allied health professionals, researchers? I am here to suggest that you add patients and the public to that list.
Next stop is commissioning research – having patients and members of the public involved in reviewing research proposals. As a funding body, a prerequisite here is that research applications include a popular science or lay summary. As a researcher, a prerequisite is that your popular science summary can be understood by patients and the public. Seek help if necessary (another opportunity for PPI!) to ensure that it is clear and easy to understand. PPI members are, of course, not limited to looking at this summary, but its presence (and quality!) does make the job easier. We are not asking them to comment on the statistical methods or whether the chosen lab technique is appropriate. We are asking them whether they think the research question is important for people with the disease, whether they think the research is feasible (are people likely to enrol?), and whether they are measuring relevant outcomes. As with most things, there are several different approaches to take in this step. Some ask PPI members to read, score, and comment on the projects in their own time using a set form. The form can cover different aspects of the project, such as the relevance of the work, the quality of the proposed work, the strength of the research team, the potential impact, value for money, and PPI plans (see here for an example from the NIHR). Personally, I am going to try a workshop where PPI members can engage in discussions about the projects. As our funding applications cover a wide variety of diagnoses, I hope that sharing experiences and perspectives across diagnoses will help PPI members to evaluate and score the applications beyond those in their specific disease.
Join me next time as we start planning and conducting our research…

1 comments