The cycle of PPI (Part II of II)
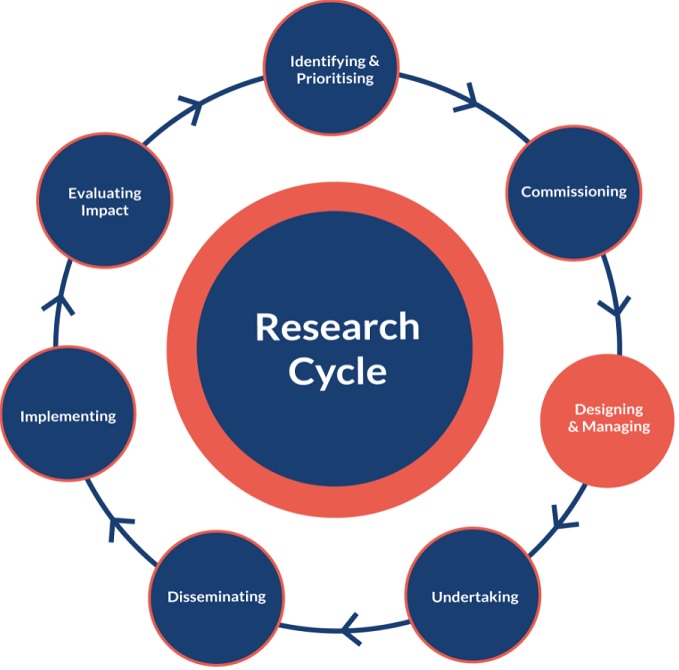
Quick recap from my previous post – PPI is important for identifying, prioritising, and commissioning research. Now we’re entering the exciting world of planning and doing the research, so let’s continue our way around the research cycle…
You’ve done it – you’ve thought up, designed, and planned a beautiful research project. It is robust and uses valid and innovative methodology. It is destined for greatness, it will change… Can I stop you right there? Are you measuring the outcomes that are important to patients or the ones that are important to you? Are you confident that participants will understand the information given to them in sufficient detail and that they will want to enrol? Just a thought, but maybe it’s worth checking that before you roll out your beautiful research project.
Now we’re coming to the researcher’s domains – starting off with designing and managing research. PPI plays a role in making sure the research is relevant, that the research question and outcomes are clear, that the recruitment process is practical and feasible, and highlighting any potential difficulties. Where you have any participant-facing documents, PPI members can help ensure that the information is user friendly, plain language, and appropriate for potential participants. For example, I had created a participant diary for my PhD project and thought that participants would prefer to have something small that could easily be carried around. But as our PPI panel pointed out – being an older age group, their priority was to have larger text that was easier to read. Recently I was told about a research project that had really been struggling with recruitment – they just couldn’t get enough people to participate and could not figure out why. They asked the opinion of some PPI members who pointed out that the study visits were scheduled first thing in the morning – too early for older people to use their free bus passes in the UK. Moving study visits to later on in the day (when participants could travel for free) was a simple fix that solved the problem with recruitment – but it took PPI members to recognise it. For my work, getting the PPI members’ thumbs up on my plan was a relief. My study was quite long and it was important to know whether they thought it was feasible before starting (turns out they were right – it was feasible!). There are many ways to go about this, but my suggestion would be to start a dialogue. Present your work (without all the jargon) and ask for their input. Circulate the documents you would be giving out to participants and ask for their feedback. Invite them in and listen to what they have to say.
You’ve sat through hours of lectures, pored through textbooks, spent hours furiously staring at code, written a thesis that proudly sits on your parent’s bookshelf – you are the expert. You are best placed to conduct, interpret, and draw conclusions from your research. Right? Hm. What if you involved other experts into the process? Experts on what it is like to live with the disease.
Now we’re at the exciting undertaking research phase and – you guessed it – you can involve patients and the public in this step too. In some projects, patients are the ones that conduct study interviews. When dealing about sensitive topics, study participants may be more likely to open to “peers” rather than research staff. Similarly, qualitative studies have invited patients and the public to identify themes in the data. When it comes to quantitative research (my world), patients and the public can help with the interpretation of the data and drawing conclusions. Once I had my results, I presented these to our patient panel and asked for their impressions. They did not go easy on me! I got quizzed (with some very valid questions), but I also heard their interpretations of the results, how they fit into their view of the disease, and their key messages. Beyond this, I also felt their reactions to the work. That emotional connection is a strong driver for me – a reminder of the importance and value of research. The input from the PPI members helped guide the story I wrote in the publication, the conclusions I drew, and the points I emphasised. Sometimes we are so bogged down in the detail of our work that it takes a fresh set of eyes and a different perspective to see its true value or how it fits into the bigger picture.
Great, research done and now you’re just faced with the question of how you disseminate your work. Easy – let’s go for a high impact journal and a conference abstract in a tropical location. Sorry, did I just say that out loud? I meant a highly relevant and informative conference. Job done. Or is it?
To maximise the impact, you need the right people to hear about it and understand it. Often, we think only of dissemination as publications in peer reviewed journals and as conference abstracts. But what about the people affected by the disease – should they not hear about this amazing work you have done for them? And the people that helped contribute to your funding, through taxes or charitable donations – should they not get to hear how you converted their funding into new knowledge? Patients and the public can be involved in this step too. On my part, our patient partners helped me form a dissemination plan and used their networks and contacts within patient organisations internationally to ensure we reached a wide audience. They helped me to ensure that the update I wrote for a patient organisation’s magazine was accessible and clear. They took part in short videos aimed at increasing awareness and informing people about our research. There really are so many ways that patient partners can contribute to the dissemination step – the first step is simply to ask their views and be open to suggestions.
Is your job truly done once you’ve saved your codes and responded to the peer reviewers’ comments? How can patients and the public help you translate theory into practice?
Finally, you have the final two steps – implementing and evaluating impact. I won’t pretend to know much about these and will not go into them in any real detail. Usually, those that are passionate enough about the research to be involved as patient partners will have the drive to help you translate your results into practice. They will be a strong ally in the implementation stage. Evaluating impact doesn’t just relate to the impact of your research, but also the impact of PPI. What worked well, what didn’t work so well, what do you need to do differently to make the most of PPI in your next project? Importantly, especially in an environment where PPI in research is not the norm, use your experience and appraisal of your PPI process to inspire and support others. I, personally, would love to learn from your stories and experiences.
Next time I will discuss some of the approaches to setting up PPI – how do you get started, where do you find people to take part, what practicalities do you need to think about, etc. Any topics you want covering? Let me know in the comments!

0 comments